Ziquen 25 / 50 / 100
- ENG
- မြန်မာ
For the use of Registered Medical Practitioner or a Hospital or Laboratory
Quetiapine Tablets 25 mg
ZIQUEN 25
Composition
Each film-coated tablet contains:
Quetiapine Fumarate
Equivalent to Quetiapine ……………….. 25 mg
Quetiapine Tablets 50 mg
ZIQUEN 50
Composition
Each film-coated tablet contains:
Quetiapine Fumarate
Equivalent to Quetiapine ……………….. 50 mg
Quetiapine Tablets 100 mg
ZIQUEN 100
Composition
Each film-coated tablet contains:
Quetiapine Fumarate
Equivalent to Quetiapine ……………….. 100 mg
1 DESCRIPTION
ZIQUEN (quetiapine fumarate) is a psychotropic agent belonging to a chemical class, the dibenzothiazepine derivatives. The chemical designation is 2-[2-(4-dibenzo [b,f] [1,4] thiazepin-11-yl-1-piperazinyl)ethoxy]-ethanol fumarate (2:1) (salt). It is present in tablets as the fumarate salt. All doses and tablet strengths are expressed as milligrams of base, not as fumarate salt. Its molecular formula is C42H50NOS₂CH₂O, and it has a molecular weight of 883.11 (fumarate salt). The structural formula is:
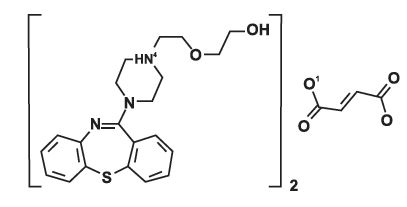
Quetiapine fumarate is a white to off-white crystalline powder which is moderately soluble in water.
2 CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism of action of QUETIAPINE is unknown. However, it has been proposed that the efficacy of QUETIAPINE in schizophrenia and its mood stabilizing properties in bipolar depression and mania are mediated through a combination of dopamine type 2 (D₂) and serotonin type 2 (5HT₂) antagonism. Antagonism at receptors other than dopamine and 5HT, with similar receptor affinities may explain some of the other effects of QUETIAPINE. QUETIAPINE’s antagonism of histamine H, receptors may explain the somnolence observed with this drug. QUETIAPINE’s antagonism of adrenergic a, receptors may explain the orthostatic hypotension observed with this drug.
3 THERAPEUTIC INDICATIONS
3.1 Schizophrenia
QUETIAPINE is indicated for the treatment of schizophrenia. The efficacy of QUETIAPINE in schizophrenia was established in three 6-week trials in adults and one 6-week trial in adolescents (13-17 years). The effectiveness of QUETIAPINE for the maintenance treatment of schizophrenia has not been systematically evaluated in controlled clinical trial.
3.2 Bipolar Disorder
QUETIAPINE is indicated for the acute treatment of manic episodes associated with bipolar I disorder, both as monotherapy and as an adjunct to lithium or divalproex. Efficacy was established in two 12-week monotherapy trials in adults, in one 3-week adjunctive trial in adults, and in one 3-week monotherapy trial in pediatric patients (10-17 years).
QUETIAPINE is indicated as monotherapy for the acute treatment of depressive episodes associated with bipolar disorder. Efficacy was established in two 8-week monotherapy trials in adult patients with bipolar I and bipolar II disorder.
QUETIAPINE is indicated for the maintenance treatment of bipolar I disorder, as an adjunct to lithium or divalproex. Efficacy was established in two maintenance trials in adults. The effectiveness of QUETIAPINE as monotherapy for the maintenance treatment of bipolar disorder has not been systematically evaluated in controlled clinical trials.
3.3 Special Considerations in Treating Pediatric Schizophrenia and Bipolar I Disorder
Pediatric schizophrenia and bipolar I disorder are serious mental disorders, however, diagnosis can be challenging. For pediatric schizophrenia, symptom profiles can be variable, and for bipolar I disorder, patients may have variable patterns of periodicity of manic or mixed symptoms. It is recommended that medication therapy for pediatric schizophrenia and bipolar I disorder be initiated only after a thorough diagnostic evaluation has been performed and careful consideration given to the risks associated with medication treatment. Medication treatment for both pediatric schizophrenia and bipolar I disorder is indicated as part of a total treatment program that often includes psychological, educational and social interventions.
4 DOSAGE AND ADMINISTRATION
4.1 Important Administration Instructions
QUETIAPINE can be taken with or without food.
4.2 Recommended Dosing
The recommended initial dose, titration, dose range and maximum QUETIAPINE dose for each approved indication is displayed in Table 1. After initial dosing, adjustments can be made upwards or downwards, if necessary, depending upon the clinical response and tolerability of the patient.
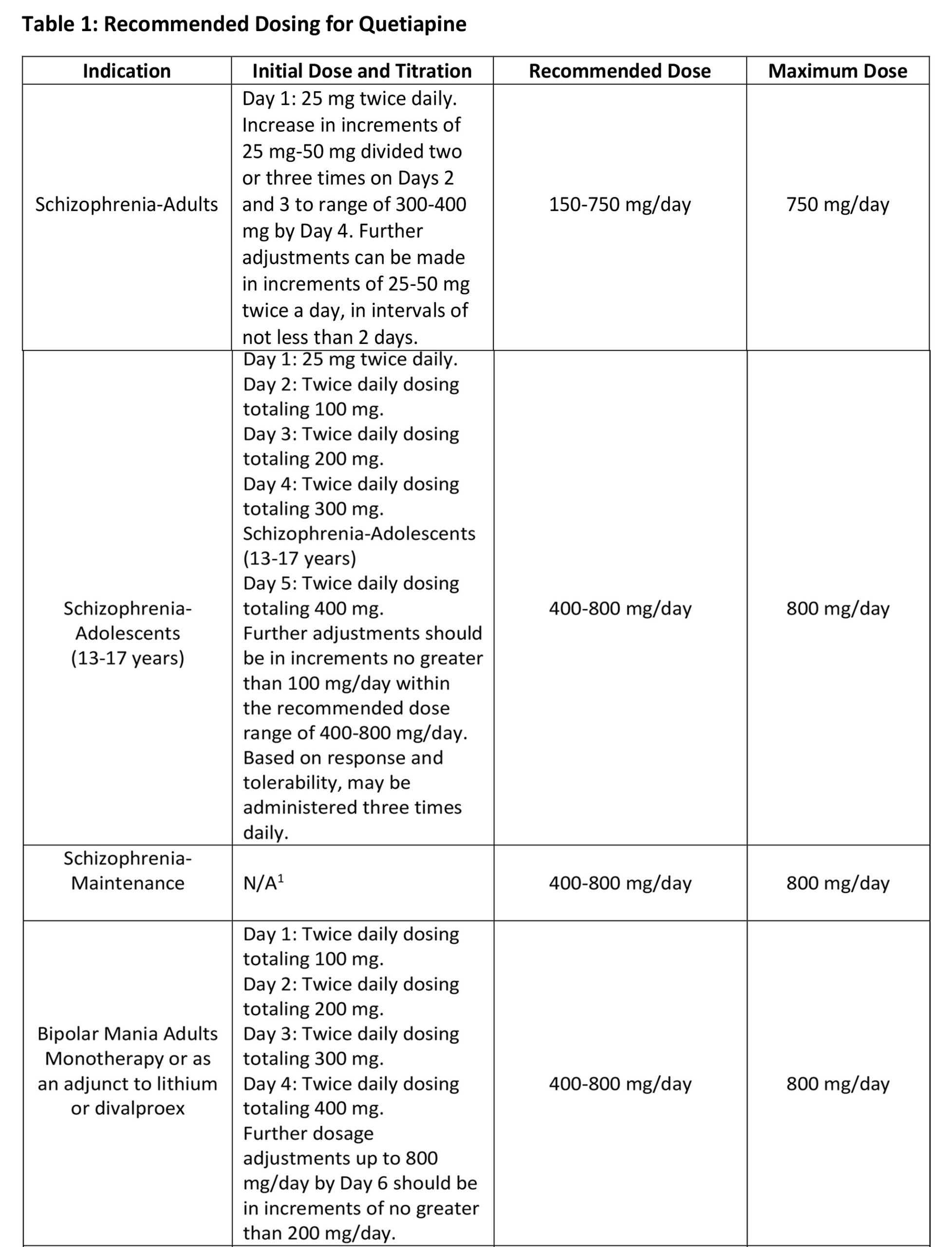
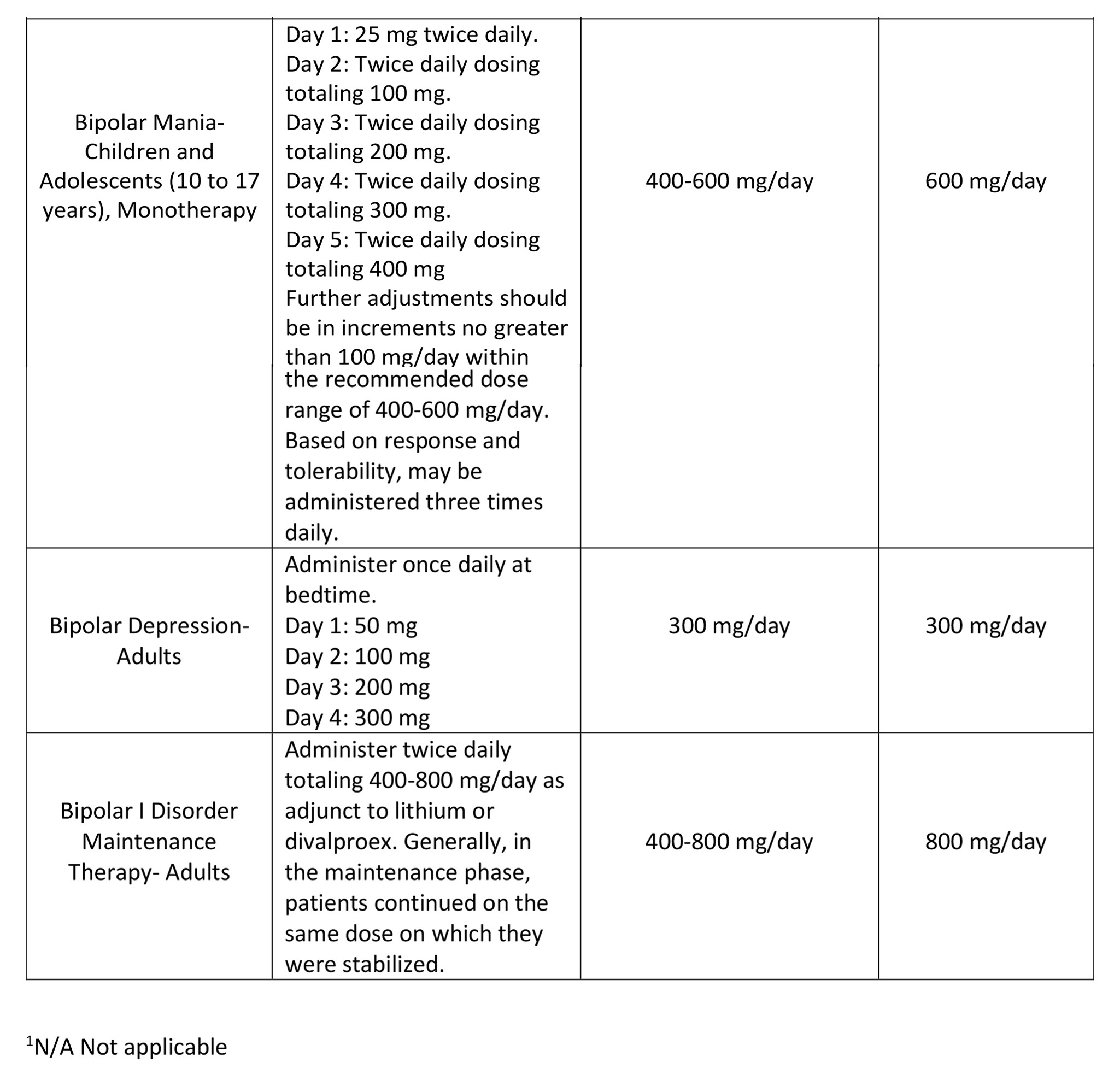
Maintenance Treatment for Schizophrenia and Bipolar I Disorder Maintenance Treatment – Patients should be periodically reassessed to determine the need for maintenance treatment and the appropriate dose for such treatment.
4.3 Dose Modifications in Elderly Patients
Consideration should be given to a slower rate of dose titration and a lower target dose in the elderly and in patients who are debilitated or who have a predisposition to hypotensive reactions. When indicated, dose escalation should be performed with caution in these patients. Elderly patients should be started on QUETIAPINE 50 mg/day and the dose can be increased in increments of 50 mg/day depending on the clinical response and tolerability of the individual patient.
4.4 Dose Modifications in Hepatically Impaired Patients
Patients with hepatic impairment should be started on 25 mg/day. The dose should be increased daily in increments of 25 mg/day – 50 mg/day to an effective dose, depending on the clinical response and tolerability of the patient.
4.5 Dose Modifications when used with CYP3A4 Inhibitors
QUETIAPINE dose should be reduced to one sixth of original dose when co-medicated with a potent CYP3A4 inhibitor (e.g., ketoconazole, itraconazole, indinavir, ritonavir, nefazodone, etc.). When the CYP3A4 inhibitor is discontinued, the dose of QUETIAPINE should be increased by 6 fold.
4.6 Dose Modifications when used with CYP3A4 Inducers
QUETIAPINE dose should be increased up to 5 fold of the original dose when used in combination with a chronic treatment (e.g., greater than 7-14 days) of a potent CYP3A4 inducer (e.g., phenytoin, carbamazepine, rifampin, avasimibe, St. John’s wort etc.). The dose should be titrated based on the clinical response and tolerability of the individual patient. When the CYP3A4 inducer is discontinued, the dose of QUETIAPINE should be reduced to the original level within 7-14 days.
4.7 Reinitiation of Treatment in Patients Previously Discontinued
Although there are no data to specifically address re-initiation of treatment, it is recommended that when restarting therapy of patients who have been off QUETIAPINE for more than one week, the initial dosing schedule should be followed. When restarting patients who have been off QUETIAPINE for less than one week, gradual dose escalation may not be required and the maintenance dose may be reinitiated.
4.8 Switching from Antipsychotics
There are no systematically collected data to specifically address switching patients with schizophrenia from antipsychotics to QUETIAPINE, or concerning concomitant administration with antipsychotics. While immediate discontinuation of the previous antipsychotic treatment may be acceptable for some patients with schizophrenia, more gradual discontinuation may be most appropriate for others. In all cases, the period of overlapping antipsychotic administration should be minimized. When switching patients with schizophrenia from depot antipsychotics, if medically appropriate, initiate QUETIAPINE therapy in place of the next scheduled injection. The need for continuing existing EPS medication should be re-evaluated periodically.
5 CONTRAINDICATIONS
Hypersensitivity to quetiapine or to any excipients in the QUETIAPINE formulation. Anaphylactic reactions have been reported in patients treated with QUETIAPINE.
6 WARNINGS AND PRECAUTIONS
– Cerebrovascular Adverse Reactions: Increased incidence of cerebrovascular adverse events (e.g., stroke, transient ischemic attack) has been seen in elderly patients with dementia-related psychoses treated with atypical antipsychotic drugs.
– Neuroleptic Malignant Syndrome (NMS): Manage with immediate discontinuation and close monitoring.
– Metabolic Changes: Atypical antipsychotics have been associated with metabolic changes. These metabolic changes include hyperglycemia, dyslipidemia, and weight gain.
– Hyperglycemia and Diabetes Mellitus: Monitor patients for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Monitor glucose regularly in patients with diabetes or at risk for diabetes.
– Dyslipidemia: Undesirable alterations have been observed in patients treated with atypical antipsychotics. Appropriate clinical monitoring is recommended, including fasting blood lipid testing at the beginning of, and periodically, during treatment.
– Weight Gain: Gain in body weight has been observed; clinical monitoring of weight is recommended.
– Tardive Dyskinesia: Discontinue if clinically appropriate
– Hypotension: Use with caution in patients with known cardiovascular or cerebrovascular disease
– Increased Blood Pressure in Children and Adolescents: Monitor blood pressure at the beginning of, and periodically during treatment in children and adolescents
– Leukopenia, Neutropenia and Agranulocytosis: Monitor complete blood count frequently during the first few months of treatment in patients with a pre-existing low white cell count or a history of leukopenia/neutropenia and discontinue QUETIAPINE at the first sign of a decline in WBC in absence of other causative factors
– Cataracts: Lens changes have been observed in patients during long-term quetiapine treatment. Lens examination is recommended when starting treatment and at 6-month intervals during chronic treatment
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS; and SUICIDAL THOUGHTS AND BEHAVIORS
Increased Mortality in Elderly Patients with Dementia-Related Psychosis.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death.
Suicidal Thoughts and Behaviors
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older.
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber.
QUETIAPINE is not approved for the treatment of patients with dementia-related psychosis.
7 ADVERSE REACTIONS
Increased mortality in elderly patients with dementia-related psychosis, Suicidal thoughts and behaviors in adolescents and young adults, Cerebrovascular adverse reactions including stroke in elderly patients with dementia-related psychosis, Neuroleptic Malignant Syndrome (NMS), Metabolic changes (hyperglycemia, dyslipidemia, weight gain), Tardive dyskinesia, Hypotension, Increases in blood pressure (children and adolescents), Leukopenia, neutropenia and agranulocytosis, Cataracts, QT Prolongation, Seizures, Hypothyroidism, Hyperprolactinemia, Potential for cognitive and motor impairment, Body temperature regulation, Dysphagia, Discontinuation Syndrome.
8 DRUG INTERACTIONS
8.1 Effect of Other Drugs on Quetiapine
The risks of using QUETIAPINE in combination with other drugs have not been extensively evaluated in systematic studies. Given the primary CNS effects of QUETIAPINE, caution should be used when it is taken in combination with other centrally acting drugs. QUETIAPINE potentiated the cognitive and motor effects of alcohol in a clinical trial in subjects with selected psychotic disorders, and alcoholic beverages should be limited while taking quetiapine.
Quetiapine exposure is increased by the prototype CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, indinavir, ritonavir, nefazodone, etc.) and decreased by the prototype CYP3A4 inducers (e.g., phenytoin, carbamazepine, rifampin, avasimibe, St. John’s wort etc.). Dose adjustment of quetiapine will be necessary if it is co-administered with potent CYP3A4 inducers or inhibitors.
CYP3A4 inhibitors:
Coadministration of ketoconazole, a potent inhibitor of cytochrome CYP3A4, resulted in significant increase in quetiapine exposure. The dose of QUETIAPINE should be reduced to one sixth of the original dose if coadministered with a strong CYP3A4.
CYP3A4 inducers:
Coadministration of quetiapine and phenytoin, a CYP3A4 inducer increased the mean oral clearance of quetiapine by 5 fold. Increased doses of QUETIAPINE up to 5 fold may be required to maintain control of symptoms of schizophrenia in patients receiving quetiapine and phenytoin, or other known potent CYP3A4. When the CYP3A4 inducer is discontinued, the dose of QUETIAPINE should be reduced to the original level within 7-14 days.
The potential effects of several concomitant medications on quetiapine pharmacokinetics were studied.
8.2 Effect of Quetiapine on Other Drugs
Because of its potential for inducing hypotension, QUETIAPINE may enhance the effects of certain antihypertensive agents.
QUETIAPINE may antagonize the effects of levodopa and dopamine agonists.
There are no clinically relevant pharmacokinetic interactions of Quetiapine on other drugs based on the CYP pathway. Quetiapine and its metabolites are non-inhibitors of major metabolizing CYP’s (1A2, 209, 2019, 2D6 and 3A4).
9 USE IN SPECIFIC POPULATIONS
9.1 Pregnancy
Pregnancy Category C:
Risk Summary
There are no adequate and well-controlled studies of QUETIAPINE use in pregnant women. In limited published literature, there were no major malformations associated with quetiapine exposure during pregnancy. In animal studies, embryo- fetal toxicity occurred. Quetiapine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
9.2 Labor and Delivery
The effect of QUETIAPINE on labor and delivery in humans is unknown.
9.3 Nursing Mothers
QUETIAPINE was excreted into human milk. Because of the potential for serious adverse reactions in nursing infants from QUETIAPINE, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother’s health.
9.4 Pediatric Use
In general, the adverse reactions observed in children and adolescents during the clinical trials were similar to those in the adult population with few exceptions. Increases in systolic and diastolic blood pressure occurred in children and adolescents and did not occur in adults. Orthostatic hypotension occurred more frequently in adults (4-7%) compared to children and adolescents (<1%). Schizophrenia: The efficacy and safety of QUETIAPINE in the treatment of schizophrenia in adolescents aged 13 to 17 years were demonstrated in one 6-week, double-blind, placebo-controlled trial. Safety and effectiveness of QUETIAPINE in pediatric patients less than 13 years of age with schizophrenia have not been established.
Maintenance: The safety and effectiveness of QUETIAPINE in the maintenance treatment of bipolar disorder has not been established in pediatric patients less than 18 years of age. The safety and effectiveness of QUETIAPINE in the maintenance treatment of schizophrenia has not been established in any patient population, including pediatric patients.
Bipolar Mania: The efficacy and safety of QUETIAPINE in the treatment of mania in children and adolescents ages 10 to 17 years with Bipolar I disorder was demonstrated in a 3-week, double-blind, placebo controlled, multicenter trial. Safety and effectiveness of QUETIAPINE in pediatric patients less than 10 years of age with bipolar mania have not been established.
Bipolar Depression: Safety and effectiveness of QUETIAPINE in
pediatric patients less than 18 years of age with bipolar depression have not been established. A clinical trial with QUETIAPINE was conducted in children and adolescents (10 to 17 years of age) with bipolar depression, efficacy was not established.
Some differences in the pharmacokinetics of quetiapine were noted between children/adolescents (10 to 17 years of age) and adults. When adjusted for weight, the AUC and Cmax of quetiapine were 41% and 39% lower, respectively, in children and adolescents compared to adults. The pharmacokinetics of the active metabolite, norquetiapine, were similar between children/adolescents and adults after adjusting for weight.
9.5 Geriatric Use
Of the approximately 3700 patients in clinical studies with QUETIAPINE, 7% (232) were 65 years of age or over. In general, there was no indication of any different tolerability of QUETIAPINE in the elderly compared to younger adults. Nevertheless, the presence of factors that might decrease pharmacokinetic clearance, increase the pharmacodynamic response to QUETIAPINE, or cause poorer tolerance or orthostasis, should lead to consideration of a lower starting dose, slower titration, and careful monitoring during the initial dosing period in the elderly. The mean plasma clearance of QUETIAPINE was reduced by 30% to 50% in elderly patients when compared to younger patients.
9.6 Renal Impairment
Clinical experience with QUETIAPINE in patients with renal impairment is limited.
9.7 Hepatic Impairment
Since quetiapine is extensively metabolized by the liver, higher plasma levels are expected in patients with hepatic impairment. In this population, a low starting dose of 25 mg/day is recommended and the dose may be increased in increments of 25 mg/day – 50 mg/day.
10 OVERDOSAGE
10.1 Human Experience
In clinical trials, survival has been reported in acute overdoses of up to 30 grams of quetiapine. Most patients who overdosed experienced no adverse reactions or recovered fully from the reported reactions. Death has been reported in a clinical trial following an overdose of 13.6 grams of quetiapine alone. In general, reported signs and symptoms were those resulting from an exaggeration of the drug’s known pharmacological effects, i.e., drowsiness and sedation, tachycardia and hypotension. Patients with pre-existing severe cardiovascular disease may be at an increased risk of the effects of overdose. One case, involving an estimated overdose of 9600 mg, was associated with hypokalemia and first degree heart block. In post-marketing experience, there were cases reported of QT prolongation with overdose. There were also very rare reports of overdose of QUETIAPINE alone resulting in death or coma.
10.2 Management of Overdosage
In case of acute overdosage, establish and maintain an airway and ensure adequate oxygenation and ventilation. Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. The possibility of obtundation, seizure or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Cardiovascular monitoring should commence immediately and should include continuous electrocardiographic monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide and quinidine carry a theoretical hazard of additive QT-prolonging effects when administered in patients with acute overdosage of QUETIAPINE. Similarly it is reasonable to expect that the alpha adrenergic-blocking properties of bretylium might be additive to those of quetiapine, resulting in problematic hypotension.
There is no specific antidote to QUETIAPINE. Therefore, appropriate supportive measures should be instituted. The possibility of multiple drug involvement should be considered. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids and/or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of quetiapine-induced alpha blockade). In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision and monitoring should continue until the patient recovers.
11 STORAGE
Store below 30°C and protect from light.
12 PRESENTATION
Alu-Alu Blister pack of 3 x 10’s in a carton along with pack insert.
KEEP MEDICINE OUT OF REACH OF CHILDREN.
Product of:
Zifam Pinnacle Pty. Ltd.,
Sydney, Australia.
Manufactured by:
Zifam Pyrex Myanmar Co. Ltd.,
Lot C6, Zone A, Thilawa SEZ, Thanlyin & Kyauk Tan Township, Yangon, Myanmar.





