Zifam Ombicar
- ENG
- မြန်မာ

For the use of Registered Medical Practitioner or a Hospital or Laboratory
Omeprazole and Sodium Bicarbonate Capsules 20 mg / 1100 mg
Zifam OMBICAR
Composition
Each capsule contains:
Omeprazole ——————— 20 mg
Sodium Bicarbonate ————— 1100 mg
DESCRIPTION
OMBICAR (omeprazole/sodium bicarbonate) is a combination of omeprazole, a proton-pump inhibitor, and sodium bicarbonate, an antacid. Omeprazole is a substituted benzimidazole, 5-methoxy-2-[[(4-methoxy-3,5- dimethyl-2-pyridinyl)methyl]sulfinyl]-1H-benzimidazole, a racemic mixture of two enantiomers that inhibits gastric acid secretion. Its empirical formula is C₁₂H₄NO₃S, with a molecular weight of 345.42. The structural formula is:
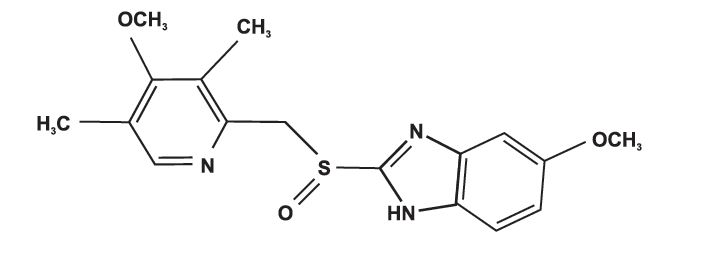
Omeprazole is a white to off-white crystalline powder which melts with decomposition at about 155°C. It is a weak base, freely soluble in ethanol and methanol, and slightly soluble in acetone and isopropanol and very slightly soluble in water. The stability of omeprazole is a function of pH; it is rapidly degraded in acid media, but has acceptable stability under alkaline conditions.
CLINICAL PHARMACOLOGY
Mechanism of Action
Omeprazole belongs to a class of antisecretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or H₂ histamine antagonistic properties, but that suppress gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, omeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus. Animal studies indicate that after rapid disappearance from plasma, omeprazole can be found within the gastric mucosa for a day or more.
Omeprazole is acid labile and thus rapidly degraded by gastric acid. OMBICAR Capsules contain sodium bicarbonate which raises the gastric pH and thus protects omeprazole from acid degradation.
THERAPEUTIC INDICATIONS
Duodenal Ulcer
OMBICAR (omeprazole/sodium bicarbonate) is indicated for short-term treatment of active duodenal ulcer. Most patients heal within four weeks. Some patients may require an additional four weeks of therapy.
Gastric Ulcer
OMBICAR is indicated for short-term treatment (4-8 weeks) of active benign gastric ulcer.
Treatment of Gastroesophageal Reflux Disease (GERD)
Symptomatic GERD
OMBICAR is indicated for the treatment of heartburn and other symptoms associated with GERD for up to 4 weeks.
Erosive Esophagitis
OMBICAR is indicated for the short-term treatment (4-8 weeks) of erosive esophagitis which has been diagnosed by endoscopy. The efficacy of OMBICAR used for longer than 8 weeks in these patients has not been established. If a patient does not respond to 8 weeks of treatment, it may be helpful to give up to an additional 4 weeks of treatment. If there is recurrence of erosive esophagitis or GERD symptoms (e.g., heartburn), additional 4-8 week courses of OMBICAR may be considered.
Maintenance of Healing of Erosive Esophagitis
OMBICAR is indicated to maintain healing of erosive esophagitis. Controlled studies do not extend beyond 12 months.
Reduction of Risk of Upper Gastrointestinal Bleeding in Critically III Patients (40mg oral suspension only)
OMBICAR is indicated for the reduction of risk of upper Gl bleeding in critically ill patients.
DOSAGE AND ADMINISTRATION
OMBICAR (omeprazole/sodium bicarbonate) is available as a capsulle in 20 mg/1100 mg omeprazolle and sodium bicarbonate for adult use. Directions for use for each indication are summarized in Table 1. All recommended doses throughout the labeling are based upon omeprazole.
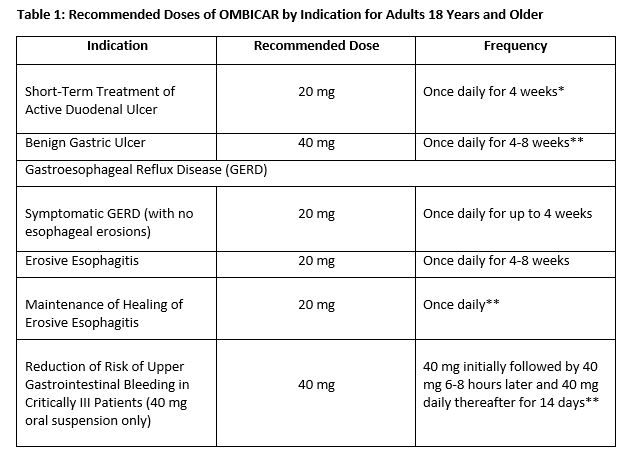
*Most patients heal within 4 weeks. Some patients may require an additional 4 weeks of therapy.
**Controlled studies do not extend beyond 12 months.
Special Populations
Hepatic Insufficiency
Consider dose reduction, particularly for maintenance of healing of erosive esophagitis.
Administration of Capsules
OMBICAR Capsules should be swallowed intact with water. DO NOT USE OTHER LIQUIDS. DO NOT OPEN CAPSULE AND SPRINKLE CONTENTS INTO FOOD.
CONTRAINDICATIONS
OMBICAR is contraindicated in patients with known hypersensitivity to any components of the formulation. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute interstitial nephritis, and urticaria.
WARNINGS AND PRECAUTIONS
Concomitant Gastric Malignancy
Symptomatic response to therapy with omeprazole does not preclude the presence of gastric malignancy.
Atrophic gastritis
Atrophic gastritis has been noted occasionally in gastric corpus biopsies from patients treated long-term with omeprazole.
Acute Interstitial Nephritis
Acute interstitial nephritis has been observed in patients taking PPIs including OMBICAR. Acute interstitial nephritis may occur at any point during PPI therapy and is generally attributed to an idiopathic hypersensitivity reaction. Discontinue OMBICAR if acute interstitial nephritis develops.
Cyanocobalamin (vitamin B-12) Deficiency
Daily treatment with any acid-suppressing medications over a long period of time (e.g., longer than 3 years) may lead to malabsorption of cyanocobalamin (vitamin B-12) caused by hypo-or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed.
Buffer Content
Each OMBICAR Capsule contains 1100 mg (13 mEq) of sodium bicarbonate.
The sodium content of OMBICAR products should be taken into consideration when administering to patients on a sodium restricted diet.
Because OMBICAR products contain sodium bicarbonate, they should be used with caution in patients with Bartter’s syndrome, hypokalemia, hypocalcemia, and problems with acid-base balance. Long-term administration of bicarbonate with calcium or milk can cause milk-alkali syndrome.
Chronic use of sodium bicarbonate may lead to systemic alkalosis and increased sodium intake can produce edema and weight increase.
Clostridium difficile Associated Diarrhea
Published observational studies suggest that PPI therapy like OMBICAR may be associated with an increased risk of Clostridium difficile associated diarrhea, especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve.
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
Interaction with Clopidogrel
Avoid concomitant use of OMBICAR with clopidogrel. Clopidogrel is a prodrug. Inhibition of platelet aggregation by clopidogrel is entirely due to an active metabolite. The metabolism of clopidogrel to its active metabolite can be impaired by use with concomitant medications, such as omeprazole, that interfere with CYP2C19 activity. Concomitant use of clopidogrel with 80 mg omeprazole reduces the pharmacological activity of clopidogrel, even when administered 12 hours apart. When using OMBICAR, consider alternative anti-platelet therapy.
Bone Fracture
Several published observational studies suggest that proton pump inhibitor (PPI) therapy may be associated with an increased risk for osteoporosis- related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to the established treatment guidelines.
Hypomagnesemia
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically.
Concomitant Use of OMBICAR with St John’s Wort or Rifampin
Drugs which induce CYP2C19 OR CYP34A (such as St John’s Wort or rifampin) can substantially decrease omeprazole concentrations. Avoid concomitant use of OMBICAR with St John’s Wort or rifampin.
Interactions with Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Providers should temporarily stop omeprazole treatment before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g. for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
Concomitant Use of OMBICAR with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients.
ADVERSE REACTIONS
The most common side effects include:
– headache
– abdominal pain
– nausea
– diarrhea
– vomiting
– gas
Serious allergic reactions:
– rash
– face swelling
– throat tightness
– difficulty breathing
DRUG INTERACTIONS
Drugs for Which Gastric pH Can Affect Bioavailability
Due to its effects on gastric acid secretion, omeprazole can reduce the absorption of drugs where gastric pH is an important determinant of their bioavailability. Like with other drugs that decrease the intragastric acidity, the absorption of drugs such as ketoconazole, atazanavir, iron salts, erlotinib, and mycophenolate mofetil (MMF) can decrease, while the absorption of drugs such as digoxin can increase during treatment with omeprazole. Concomitant treatment with omeprazole (20 mg daily) and digoxin in healthy subjects increased the bioavailability of digoxin by 10% (30% in two subjects). Coadministration of digoxin with OMBICAR is expected to increase the systemic exposure of digoxin. Therefore, patients may need to be monitored when digoxin is taken concomitantly with OMBICAR.
Co-administration of omeprazole in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving OMBICAR and MMF. Use OMBICAR with caution in transplant patients receiving MMF.
Drugs Metabolized by Cytochrome P450 (CYP)
Omeprazole can prolong the elimination of diazepam, warfarin and phenytoin, drugs that are metabolized by oxidation in the liver. There have been reports of increased INR and prothrombin time in patients receiving proton pump inhibitors, including omeprazole, and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with proton pump inhibitors and warfarin may need to be monitored for increases in INR and prothrombin time.
Although in normal subjects no interaction with theophylline or propranolol was found, there have been clinical reports of interaction with other drugs metabolized via the cytochrome P-450 system (e.g., cyclosporine, disulfiram, benzodiazepines). Patients should be monitored to determine if it is necessary to adjust the dosage of these drugs when taken concomitantly with OMBICAR.
Concomitant administration of omeprazole and voriconazole (a combined inhibitor of CYP2C19 and CYP3A4) resulted in more than doubling of the omeprazole exposure. Dose adjustment of omeprazole is not normally required. When voriconazole (400 mg every 12 hours for one day, then 200 mg for 6 days) was given with omeprazole (40 mg once daily for 7 days) to healthy subjects, it significantly increased the steady-state Cmax and AUC0-24 of omeprazole, an average of 2 times (90% CI: 1.8, 2.6) and 4 times (90% CI: 3.3, 4.4) respectively as compared to when omeprazole was given without voriconazole.
Drugs known to induce CYP2C19 or CYP3A4 (such as rifampin) may lead to decreased omeprazole serum levels. In a cross-over study in 12 healthy male subjects, St John’s wort (300 mg three times daily for 14 days), an inducer of CYP3A4, decreased the systemic exposure of omeprazole in CYP2C19 poor metabolisers (Cmax and AUC decreased by 37.5% and 37.9%, respectively) and extensive metabolisers (Cmax and AUC decreased by 49.6% and 43.9%, respectively). Avoid concomitant use of St. John’s Wort or rifampin with omeprazole.
Antiretroviral Agents
Concomitant administration of atazanavir and proton pump inhibitors is not recommended. Co-administration of atazanavir with proton pump inhibitors is expected to substantially decrease atazanavir plasma concentrations and thereby reduce its therapeutic effect.
Omeprazole has been reported to interact with some antiretroviral drugs. The clinical importance and the mechanisms behind these interactions are not always known. Increased gastric pH during omeprazole treatment may change the absorption of the antiretroviral drug. Other possible interaction mechanisms are via CYP2C19. For some antiretroviral drugs, such as atazanavir and nelfinavir, decreased serum levels have been reported when given together with omeprazole. Following multiple doses of nelfinavir (1250 mg, twice daily) and omeprazole (40 mg, daily), AUC was decreased by 36% and 92%, Cmax by 37% and 89% and Cmin by 39% and 75% respectively for nelfinavir and M8. Following multiple doses of atazanavir (400 mg, daily) and omeprazole (40 mg, dailly, 2 hours before atazanavir), AUC was decreased by 94%, Cmax by 96%, and Cmin by 95%. Concomitant administration with omeprazole and drugs such as atazanavir and nelfinavir is therefore not recommended.
Increased Concentration of Saquinavir
For other antiretroviral drugs, such as saquinavir, elevated serum levels have been reported with an increase in AUC by 82%, in Cmax by 75% and in Cmin by 106% following multiple dosing of saquinavir/ritonavir (1000/100 mg) twice daily for 15 days with omeprazole 40 mg dailly co-administered days 11 to 15. Dose reduction of saquinavir should be considered from the safety perspective for individual patients. There are also some antiretroviral drugs of which unchanged serum levels have been reported when given with omeprazole.
Combination Therapy with Clarithromycin
Concomitant administration of clarithromycin with other drugs can lead to serious adverse reactions due to drug interaction. Because of these drug interactions, clarithromycin is contraindicated for co-administration with certain drugs.
Clopidogrel
Omeprazole is an inhibitor of CYP2C19 enzyme. Clopidogrel is metabolized to its active metabolite in part by CYP2C19. Concomitant use of omeprazole 80 mg results in reduced plasma concentrations of the active metabolite of clopidogrel and a reduction in platelet inhibition. Avoid concomitant administration of OMBICAR with clopidogrel. When using OMBICAR, consider use of alternative anti-platelet therapy.
Tacrolimus
Concomitant administration of omeprazole and tacrolimus may increase the serum levels of tacrolimus.
Interactions With Investigations of Neuroendocrine Tumors
Drug-induced decrease in gastric acidity results in enterochromaffin-like cell hyperplasia and increased Chromogranin A levels which may interfere with investigations for neuroendocrine tumors.
Methotrexate
Case reports, published population pharmacokinetic studies, and retrospective analyses suggest that concomitant administration of PPIs and methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate. However, no formal drug interaction studies of methotrexate with PPIs have been conducted.
USE IN SPECIFIC POPULATIONS
Pregnancy
Pregnancy Category C Risk Summary
There are no adequate and well-controlled studies on the use of OMBICAR in pregnant women. Available epidemiologic data fail to demonstrate an increased risk of major congenital malformations or other adverse pregnancy outcomes with first trimester omeprazole use. Teratogenicity was not observed in animal reproduction studies with administration of oral esomeprazole magnesium in rats and rabbits with doses about 68 times and 42 times, respectively, an oral human dose of 40 mg (based on a body surface area basis for a 60 kg person). However, changes in bone morphology were observed in offspring of rats dosed through most of pregnancy and lactation at doses equal to or greater than approximately 33.6 times an oral human dose of 40 mg. Because of the observed effect at high doses of esomeprazole magnesium on developing bone in rat studies, OMBICAR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Omeprazole concentrations have been measured in breast milk of a woman following oral administration of 20 mg. The peak concentration of omeprazole in breast milk was less than 7% of the peak serum concentration. The concentration will correspond to 0.004 mg of omeprazole in 200 mL of milk. Because omeprazole is excreted in human milk, because of the potential for serious adverse reactions in nursing infants from omeprazole, and because of the potential for tumorigenicity shown for omeprazole in rat carcinogenicity studies, a decision should be made to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In addition, sodium bicarbonate should be used with caution in nursing mothers.
Pediatric Use
Safety and effectiveness of OMBICAR have not been established in pediatric patients less than 18 years of age.
Geriatric Use
Omeprazole was administered to over 2000 elderly individuals (≥ 65 years of age) in clinical trials in the U.S. and Europe. There were no differences in safety and effectiveness between the elderly and younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Pharmacokinetic studies with buffered omeprazole have shown the elimination rate was somewhat decreased in the elderly and bioavailability was increased. The plasma clearance of omeprazole was 250 mL/min (about half that of young subjects). The plasma half-life averaged one hour, about twice that in nonelderly, healthy subjects taking OMBICAR. However, no dosage adjustment is necessary in the elderly.
Hepatic Impairment
Consider dose reduction, particularly for maintenance of healing of erosive esophagitis.
Renal Impairment
No dose reduction is necessary.
Asian Population
Recommend dose reduction, particularly for maintenance of healing of erosive esophagitis.
OVERDOSAGE
Reports have been received of over dosage with omeprazole in humans. Doses ranged up to 2400 mg (120 times the usual recommended clinical dose).
Manifestations were variable, but included confusion, drowsiness, blurred vision, tachycardia, nausea, vomiting, diaphoresis, flushing, headache, dry mouth, and other adverse reactions similar to those seen in normal clinical experience. Symptoms were transient, and no serious clinical outcome has been reported when omeprazole was taken alone. No specific antidote for omeprazole over dosage is known. Omeprazole is extensively protein bound and is, therefore, not readily dialyzable. In the event of over dosage, treatment should be symptomatic and supportive. Single oral doses of omeprazole at 1350, 1339, and 1200 mg/kg were lethal to mice, rats, and dogs, respectively. Animals given these doses showed sedation, ptosis, tremors, convulsions, and decreased activity, body temperature, and respiratory rate and increased depth of respiration.
In addition, a sodium bicarbonate overdose may cause hypocalcemia, hypokalemia, hypernatremia, and seizures.
STORAGE
Store below 30°C and protect from light.
PRESENTATION
Alu-Alu Blister pack of 3 x 10’s in a carton along with pack insert.
KEEP MEDICINE OUT OF REACH OF CHILDREN.
Product of:
Zifam Pinnacle Pty. Ltd.,
Sydney, Australia.
Manufactured by:
Zifam Pyrex Myanmar Co. Ltd.,
Lot C6, Zone A, Thilawa SEZ, Thanlyin & Kyauk Tan Township, Yangon, Myanmar.