Zicito 10 / 20
- ENG
- မြန်မာ

For the use of Registered Medical Practitioner or a Hospital or Laboratory
Citalopram Tablets 10 mg
ZICITO 10
Composition
Each film-coated tablet contains:
Citalopram Hydrobromide
Equivalent to Citalopram ……………… 10 mg
Citalopram Tablets ……………… 20 mg
ZICITO 20
Composition
Each film-coated tablet contains:
Citalopram Hydrobromide
Equivalent to Citalopram ……………… 20 mg
1 DESCRIPTION
ZICITO contains Citalopram, a selective serotonin reuptake inhibitor (SSRI). Citalopram hydrobromide is a racemic bicyclic phthalane structure and is designated (+)-1-(3-dimethylaminopropyl)-1-(4-fluorophenyl)-1, 3dihydroisobenzofuran-5-carbonitrille hydrobromide with the following structural formula:
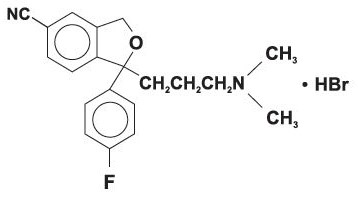
The molecular formula is C20H22BrFN₂O and its molecular weight is 405.35.
Citalopram hydrobromide occurs as a fine, white to off-white powder. Citalopram hydrobromide is sparingly soluble in water and soluble in ethanol.
2 CLINICAL PHARMACOLOGY
2.1 Mechanism of Action
The mechanism of action of Citalopram is unclear, but is presumed to be related to potentiation of serotonergic activity in the central nervous system (CNS) resulting from its inhibition of CNS neuronal reuptake of serotonin (5-HT).
3 THERAPEUTIC INDICATIONS
Citalopram is indicated for the treatment of major depressive disorder (MDD) in adults.
4 DOSAGE AND ADMINISTRATION
4.1 Recommended Dosage
Administer Citalopram once daily, with or without food, at an initial dosage of 20 mg once daily, with an increase to a maximum dosage of 40 mg once daily at an interval of not less than one week. Dosages above 40 mg once daily are not recommended due to the risk of QT prolongation.
4.2 Screen for Bipolar Disorder Prior to Starting CITALOPRAM
Prior to initiating treatment with Citalopram or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania.
4.3 Recommended Dosage for Specific Populations
The maximum recommended dosage of Citalopram for patients who are greater than 60 years of age, patients with hepatic impairment, and for CYP2C19 poor metabolizers is 20 mg once daily.
4.4 Dosage Modifications with Concomitant Use of CYP2C19 Inhibitors
The maximum recommended dosage of Citalopram when used concomitantly with a CYP2C₁ inhibitor is 20 mg once daily.
4.5 Switching Patients to or from a Monoamine Oxidase Inhibitor Antidepressant
At least 14 days must elapse between discontinuation of a monoamine oxidase inhibitor (MAOI) antidepressant and initiation of therapy with Citalopram. Conversely, at least 14 days must elapse after stopping Citalopram before starting an MAOI antidepressant.
4.6 Discontinuing Treatment with CITALOPRAM
Adverse reactions may occur upon discontinuation of Citalopram. Gradually reduce the dosage rather than stopping Citalopram abruptly whenever possible.
5 CONTRAINDICATIONS
Citalopram is contraindicated in patients:
taking, or within 14 days of stopping, MAOIs (including MAOIs such as linezolid or intravenous methylene blue) because of an increased risk of serotonin syndrome.
taking pimozide because of risk of QT prolongation.
with known hypersensitivity to Citalopram or any of the inactive ingredients in Citalopram. Reactions have included angioedema and anaphylaxis.
6 WARNINGS AND PRECAUTIONS
6.1 Suicidal Thoughts and Behavior in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients, and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.
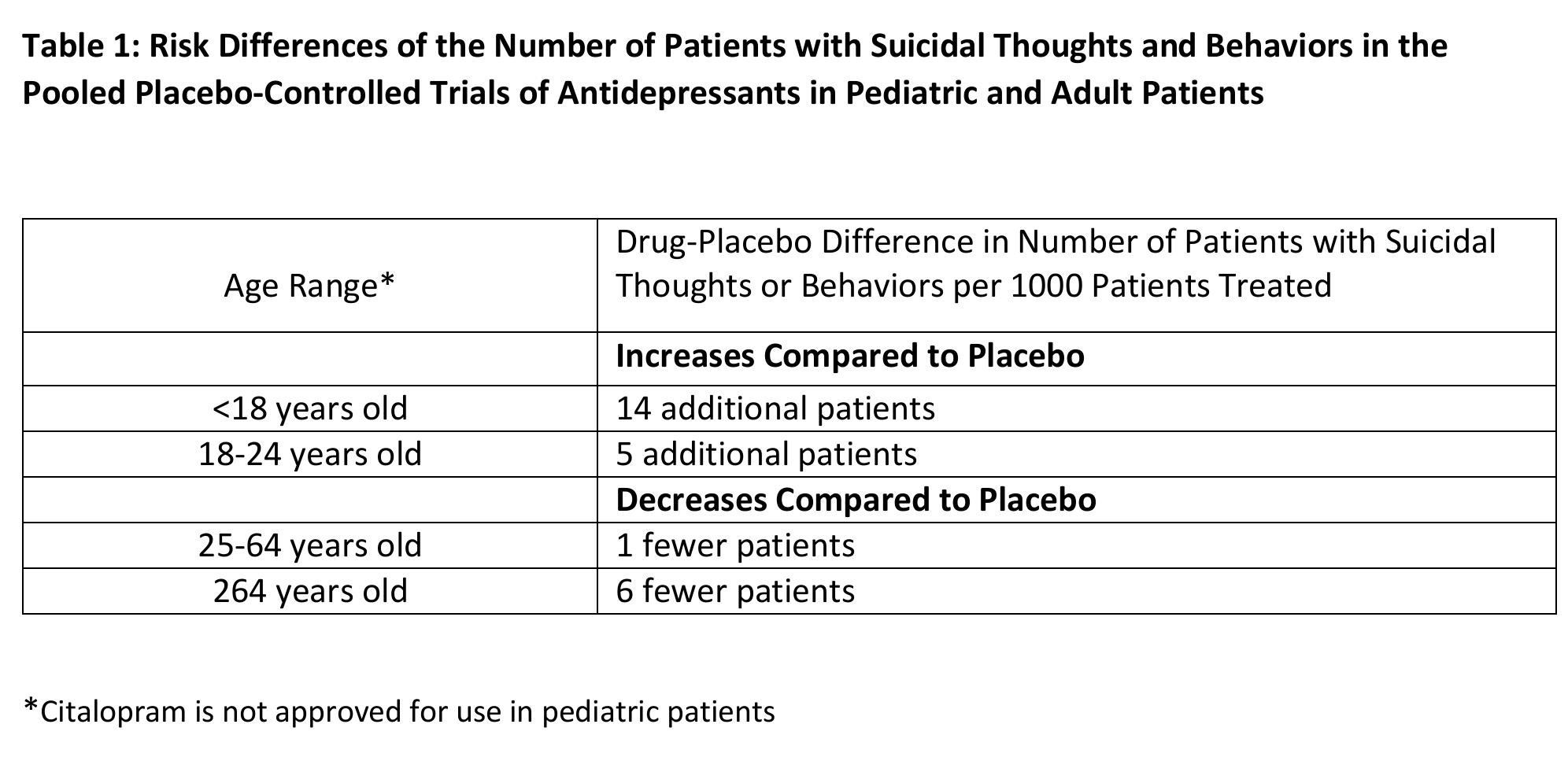
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors. Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing CITALOPRAM, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
6.2 QT-Prolongation and Torsade de Pointes
CITALOPRAM causes dose-dependent QTc prolongation an ECG abnormality that has been associated with Torsade de Pointes (TdP), ventricular tachycardia, and sudden death, all of which have been observed in postmarketing reports for citalopram.
Because of the risk of QTc prolongation at higher CITALOPRAM doses, it is recommended that CITALOPRAM not be given at doses above 40 mg once daily.
CITALOPRAM should be avoided in patients with congenital long QT syndrome, bradycardia, hypokalemia or hypomagnesemia, recent acute myocardial infarction, or uncompensated heart failure unless the benefits outweigh the risks for a particular patient.
CITALOPRAM should also be avoided in patients who are taking other drugs that prolong the QTc interval. Such drugs include Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval (e.g., pentamidine, levomethadyl acetate, methadone).
The citalopram dose should be limited in certain populations. The maximum dose should be limited to 20 mg once daily in patients who are CYP2C19 poor metabolizers or those patients receiving concomitant cimetidine or another CYP2C19 inhibitor, since higher citalopram exposures would be expected. The maximum dose should also be limited to 20 mg once daily in patients with hepatic impairment and in patients who are greater than 60 years of age because of expected higher exposures.
Electrolyte and/or ECG monitoring is recommended in certain circumstances. Patients being considered for treatment with CITALOPRAM who are at risk for significant electrolyte disturbances should have baseline serum potassium and magnesium measurements with periodic monitoring. Hypokalemia (and/or hypomagnesemia) may increase the risk of QTc prolongation and arrhythmia, and should be corrected prior to initiation of treatment and periodically monitored. ECG monitoring is recommended in patients for whom CITALOPRAM use is not recommended unless the benefits clearly outweigh the risks for a particular patient. These include those patients with the cardiac conditions noted above, and those taking other drugs that may prolong the QTc interval.
Discontinue CITALOPRAM in patients who are found to have persistent QTc measurements >500 ms. If patients taking CITALOPRAM experience symptoms that could indicate the occurrence of cardiac arrhythmias, e.g., dizziness, palpitations, or syncope, the prescriber should initiate further evaluation, including cardiac monitoring.
6.3 Serotonin Syndrome
SSRIs, including CITALOPRAM, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs. Serotonin syndrome can also occur when these drugs are used alone. Symptoms of serotonin syndrome were noted in 0.1% of MDD patients treated with CITALOPRAM in premarketing clinical trials.
Serotonin syndrome signs and symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of CITALOPRAM with MAOIS is contraindicated. In addition, do not initiate CITALOPRAM in a patient being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking CITALOPRAM, discontinue CITALOPRAM before initiating treatment with the MAOI.
Monitor all patients taking CITALOPRAM for the emergence of serotonin syndrome. Discontinue treatment with CITALOPRAM and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of CITALOPRAM with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
6.4 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including CITALOPRAM, increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS), other antiplatelet drugs, warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to drugs that interfere with serotonin reuptake have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the increased risk of bleeding associated with the concomitant use of CITALOPRAM and antiplatelet agents or anticoagulants. For patients taking warfarin, carefully monitor the international normalized ratio.
6.5 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with CITALOPRAM or another antidepressant may precipitate a mixed/manic episode. In controlled clinical trials, patients with bipolar disorder were excluded; however, symptoms of mania or hypomania were reported in 0.1% of undiagnosed patients treated with CITALOPRAM. Prior to initiating treatment with CITALOPRAM, screen patients for any personal or family history of bipolar disorder, mania, or hypomania.
6.6 Discontinuation Syndrome
Adverse reactions after discontinuation of serotonergic antidepressants, particularly after abrupt discontinuation, include: nausea, sweating, dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), tremor, anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. A gradual reduction in dosage rather than abrupt cessation is recommended whenever possible.
6.7 Seizures
CITALOPRAM has not been systematically evaluated in patients with seizure disorders. Patients with a history of seizures were excluded from clinical studies. In clinical trials of CITALOPRAM, seizures occurred in 0.3% of patients treated with CITALOPRAM (a rate of one patient per 98 years of exposure) and 0.5% of patients treated with placebo (a rate of one patient per 50 years of exposure). CITALOPRAM should be prescribed with caution in patients with a seizure disorder.
6.8 Angle-closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs, including CITALOPRAM, may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Avoid use of antidepressants, including CITALOPRAM, in patients with untreated anatomically narrow angles.
6.9 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs, including CITALOPRAM. Cases of serum sodium lower than 110 mmol/L have been reported. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
In patients with symptomatic hyponatremia, discontinue CITALOPRAM and institute appropriate medical intervention. Elderly patients, patients taking diuretics, and those who are volume-depleted may be at greater risk of developing hyponatremia with SSRIs.
6.10 Sexual Dysfunction
Use of SSRIs, including CITALOPRAM, may cause symptoms of sexual dysfunction. In male patients, SSRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SSRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of CITALOPRAM and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment.
7 ADVERSE REACTIONS
– Hypersensitivity reactions
– Suicidal thoughts and behaviors in adolescents and young adults
– QT-prolongation and torsade de pointes
– Serotonin syndrome
– Increased risk of bleeding
– Activation of mania or hypomania
– Discontinuation syndrome
– Seizures
– Angle-closure glaucoma
– Hyponatremia
– Sexual Dysfunction
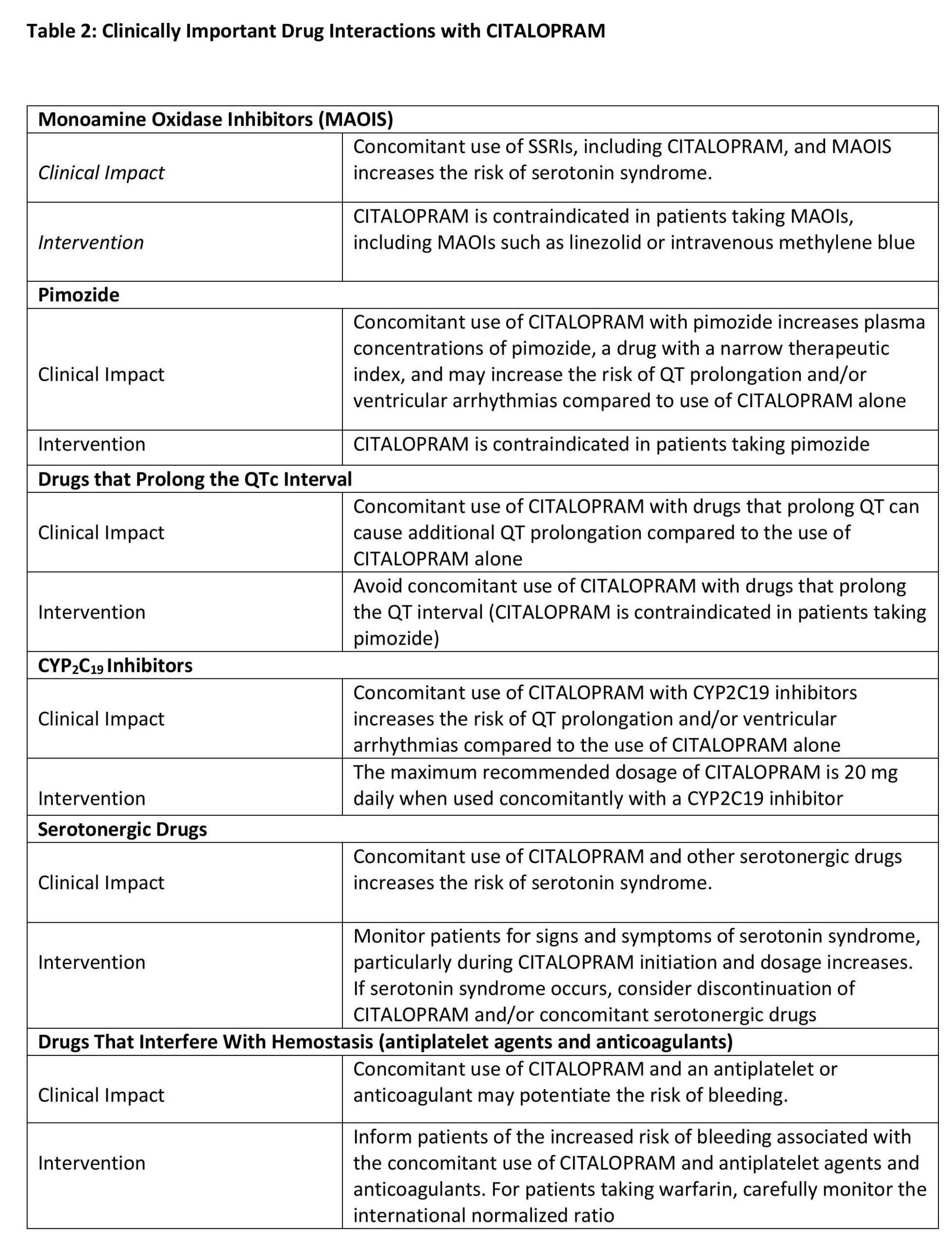
8 DRUGINTERACTIONS
Table 2 presents clinically important drug interactions with CITALOPRAM.
9 USE IN SPECIFIC POPULATIONS
9.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy.
Risk Summary
Available data from published epidemiologic studies and post-marketing reports with citalopram use in pregnancy have not established an increased risk of major birth defects or miscarriage.
Published studies demonstrated that citalopram levels in both cord blood and amniotic fluid are similar to those observed in maternal serum. There are risks of persistent pulmonary hypertension of the newborn (PPHN) and/or poor neonatal adaptation with exposure to selective serotonin reuptake inhibitors (SSRIs), including CITALOPRAM, during pregnancy. There also are risks associated with untreated depression in pregnancy.
In animal reproduction studies, citalopram caused adverse embryo/fetal effects at doses that caused maternal toxicity.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in the clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Women who discontinue antidepressants during pregnancy are more likely to experience a relapse of major depression than women who continue antidepressants. This finding is from a prospective longitudinal study of 201 pregnant women with a history of major depressive disorder who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and postpartum.
Fetal/Neonatal Adverse Reactions
Neonates exposed to CITALOPRAM and other SSRIs late in third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These findings are consistent with either a direct toxic effect of SSRIs or possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome.
Data
Human Data
Exposure during late pregnancy to SSRIs may have an increased risk for persistent pulmonary hypertension of the newborn (PPHN). PPHN occurs in 1-2 per 1,000 live births in the general population and is associated with substantial neonatal morbidity and mortality.
Animal Data
Citalopram was administered orally to pregnant rats during the period of organogenesis at doses of 32, 56, and 112 mg/kg/day, which are approximately 8, 14, and 27 times the Maximum Recommended Human Dose (MRHD) of 40 mg, based on mg/m2 body surface area. Citalopram caused maternal toxicity of CNS clinical signs and decreased weight gain at 112 mg/kg/day, which is 27 times the MRHD. At this maternally toxic dose, citalopram decreased embryo/fetall growth and survival and increased fetal abnormalities (including cardiovascular and skeletal defects). The no observed adverse effect level (NOAEL) for maternal and embryofetal toxicity is 56 mg/kg/day, which is approximately 14 times the MRHD.
Citalopram was administered orally to pregnant rats during late gestation and lactation periods at doses of 4.8, 12.8, and 32 mg/kg/day, which are approximately 1, 3, and 8 times the MRHD of 40 mg, based on mg/m2 body surface area.
Citalopram increased offspring mortality during the first 4 days of birth and decreased offspring growth at 32 mg/kg/day, which is approximately 8 times the MRHD. The NOAEL for developmental toxicity is 12.8 mg/kg/day, which is approximately 3 times the MRHD. In a separate study, similar effects on offspring mortality and growth were seen when dams were treated throughout gestation and early lactation at doses ≥ 24 mg/kg/day, which is approximately 6 times the MRHD. ANOAEL was not determined in that study.
9.2 Lactation
Risk Summary
Data from the published literature report the presence of citalopram in human milk at relative infant doses ranging between 0.7 to 9.4% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 0.78 to 4.3. There are reports of breastfed infants exposed to citalopram experiencing irritability, restlessness, excessive somnolence, decreased feeding, and weight loss. There is no information about effects of citalopram on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for CITALOPRAM and any potential adverse effects on the breastfed child from CITALOPRAM or from the underlying maternal condition.
Clinical Considerations
Monitor breastfeeding infants for adverse reactions, such as irritability, restlessness, excessive somnolence, decreased feeding, and weight loss.
9.3 Pediatric Use
The safety and effectiveness of CITALOPRAM have not been established in pediatric patients. Two placebo-controlled trials in 407 pediatric patients with MDD have been conducted with CITALOPRAM, and the data were not sufficient to support use in pediatric patients.
Antidepressants increase the risk of suicidal thoughts and behaviors in pediatric patients. Decreased appetite and weight loss have been observed in association with the use of SSRIs in pediatric patients.
9.4 Geriatric Use
Of 4422 patients in clinical studies of CITALOPRAM, 1357 were 60 and over, 1034 were 65 and over, and 457 were 75 and over. In two pharmacokinetic studies, citalopram AUC was increased by 23% and 30%, respectively, in subjects 60 years of age as compared to younger subjects, and its half-life was increased by 30% and 50%, respectively. Therefore, the maximum recommended dosage in patients 60 years of age and older is lower than younger patients.
SSRIs, including CITALOPRAM, have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse reaction.
10 OVERDOSAGE
The following have been reported with Citalopram tablet overdosage:
– Seizures, which may be delayed, and altered mental status including coma.
– Cardiovascular toxicity, which may be delayed, including QRS and QTc interval prolongation, wide complex tachyarrhythmias, and torsade de pointes. Hypertension most commonly seen, but rarely can see hypotension alone or with co-ingestants including alcohol.
– Serotonin syndrome (patients with a multiple drug overdosage with other proserotonergic drugs may have a higher risk).
Prolonged cardiac monitoring is recommended in Citalopram overdosage ingestions due to the arrhythmia risk.
Gastrointestinal decontamination with activated charcoal should be considered in patients who present early after a Citalopram overdose.
11 STORAGE
Store below 30°C and protect from light.
12 PRESENTATION
Alu-Alu blister pack of 3 x 10’s in a carton along with pack insert.
KEEP MEDICINE OUT OF REACH OF CHILDREN.
Product of:
Zifam Pinnacle Pty. Ltd.,
Sydney, Australia.
Manufactured by:
Zifam Pyrex Myanmar Co. Ltd.,
Lot C6, Zone A, Thilawa SEZ, Thanlyin & Kyauk Tan Township, Yangon, Myanmar.





