Grace Letrozole
- ENG
- မြန်မာ

For the use only of a registered medical practitioner or a hospital or a laboratory
Rx
GRACE LETROZOLE
Letrozole Tablets USP 2.5mg
COMPOSITION
Each film coated tablet contains:
Letrozole USP 2.5mg
Excipients q.s.
PHARMACEUTICAL FORM
Film coated tablet
THERAPEUTIC INDICATIONS
– Adjuvant treatment of postmenopausal women with hormone receptor positive invasive early breast cancer.
– Extended adjuvant treatment of hormone-dependent invasive breast cancer in postmenopausal women who have received prior standard adjuvant tamoxifen therapy for 5 years.
– First-line treatment in postmenopausal women with hormone-dependent advanced breast cancer.
– Advanced breast cancer after relapse or disease progression, in women with natural or artificially induced postmenopausal endocrine status, who have previously been treated with anti-oestrogens.
– Neo-adjuvant treatment of postmenopausal women with hormone receptor positive, HER-2 negative breast cancer where chemotherapy is not suitable and immediate surgery not indicated.
Efficacy has not been demonstrated in patients with hormone receptor negative breast cancer.
POSOLOGYAND METHOD OFADMINISTRATION
Posology
Adult and elderly patients
The recommended dose of Letrozole Tablets USP, 2.5 mg is 2.5 mg once daily. No dose adjustment is required for elderly patients.
In patients with advanced or metastatic breast cancer, treatment with Letrozole Tablets USP, 2.5 mg should continue until tumour progression is evident.
In the adjuvant and extended adjuvant setting, treatment with Letrozole Tablets USP, 2.5 mg should continue for 5 years or until tumour relapse occurs, whichever is first.
In the adjuvant setting a sequential treatment schedule (letrozole 2 years followed by tamoxifen 3 years) could also be considered.
In the neoadjuvant setting, treatment with Letrozole Tablets USP, 2.5 mg could be continued for 4 to 8 months in order to establish optimal tumour reduction. If the response is not adequate, treatment with Letrozole Tablets USP, 2.5 mg should be discontinued and surgery scheduled and/or further treatment options discussed with the patient.
Paediatric population
Letrozole Tablets USP, 2.5 mg is not recommended for use in children and adolescents. The safety and effiicacy of Letrozole Tablets USP, 2.5 mg in children and adolescents aged up to 17 years have not been established. Limited data are available and no recommendation on a posology can be made.
Renal impairment
No dosage adjustment of Letrozole Tablets USP, 2.5 mg is required for patients with renal insufficiency with creatinine clearance ≥10 ml/min. Insufficient data are available in cases of renal insufficiency with creatinine clearance lower than 10 ml/min.
Hepatic impairment
No dose adjustment of Letrozole Tablets USP, 2.5 mg is required for patients with mild to moderate hepatic insufficiency (Child-Pugh A or B). Insufficient data are available for patients with severe hepatic impairment. Patients with severe hepatic impairment (Child-Pugh C) require close supervision.
Method of administration
Letrozole Tablets USP, 2.5 mg should be taken orally and can be taken with or without food.
A missed dose should be taken as soon as the patient remembers. However, if it is almost time for the next dose (within 2 or 3 hours), the missed dose should be skipped, and the patient should go back to her regular dosage schedule. Doses should not be doubled because with daily doses over the 2.5 mg recommended dose, over-proportionality in systemic exposure was observed.
CONTRAINDICATIONS
– Hypersensitivity to the active substance or to any of the excipients listed
– Premenopausal endocrine status
– Pregnancy
– Breast-feeding
WARNINGS AND PRECAUTIONS
Menopausal status
In patients whose menopausal status is unclear, luteinising hormone (LH), follicle-stimulating hormone (FSH) and/or oestradiol levels should be measured before initiating treatment with Letrozole Tablets USP, 2.5 mg. Only women of postmenopausal endocrine status should receive Letrozole Tablets USP, 2.5 mg.
Renal impairment
Letrozole Tablets USP, 2.5 mg has not been investigated in a sufficient number of patients with a creatinine clearance lower than 10 ml/min. The potential risk/benefit to such patients should be carefully considered before administration of Letrozole Tablets USP, 2.5 mg.
Hepatic impairment
In patients with severe hepatic impairment (Child-Pugh C), systemic exposure and terminal half-life were approximately doubled compared to healthy volunteers. Such patients should therefore be kept under close supervision.
Bone effects
Letrozole Tablets USP, 2.5 mg is a potent oestrogen-lowering agent. Women with a history of osteoporosis and/or fractures, or who are at increased risk of osteoporosis, should have their bone mineral density formally assessed prior to the commencement of adjuvant and extended adjuvant treatment and monitored during and following treatment with letrozole. Treatment or prophylaxis for osteoporosis should be initiated as appropriate and carefully monitored. In the adjuvant setting a sequential treatment schedule (letrozole 2 years followed by tamoxifen 3 years) could also be considered depending on the patient’s safety profile.
Other warnings
Co-administration of Letrozole Tablets USP, 2.5 mg with tamoxifen, other anti-oestrogens or oestrogen-containing therapies should be avoided as these substances may diminish the pharmacological action of Letrozole.
As the tablets contain lactose, Letrozole Tablets USP, 2.5 mg is not recommended for patients with rare hereditary problems of galactose intolerance, of severe lactase deficiency or of glucose-galactose malabsorption.
DRUG INTERACTIONS
Metabolism of letrozole is partly mediated via CYP2A6 and CYP3A4. Cimetidine, a weak, unspecic inhibitor of CYP450 enzymes, did not affect the plasma concentrations of letrozole. The effect of potent CYP450 inhibitors is unknown.
There is no clinical experience to date on the use of Letrozole Tablets USP, 2.5 mg in combination with oestrogens or other anticancer agents, other than tamoxifen. Tamoxifen, other anti-oestrogens or oestrogen-containing therapies may diminish the pharmacological action of letrozole. In addition, co-administration of tamoxifen with letrozole has been shown to substantially decrease plasma concentrations of letrozole. Co-administration of letrozole with tamoxifen, other anti-oestrogens or oestrogens should be avoided.
In vitro, letrozole inhibits the cytochrome P450 isoenzymes 2A6 and, moderately, 2C19, but the clinical relevance is unknown. Caution is therefore indicated when giving letrozole concomitantly with medicinal products whose elimination is mainly dependent on these isoenzymes and whose therapeutic index is narrow (e.g. phenytoin, clopidrogel).
FERTILITY PREGNANCYAND LACTATION
Pregnancy
Based on human experience in which there have been isolated cases of birth defects (labial fusion, ambiguous genitalia), Letrozole Tablets USP, 2.5 mg may cause congenital malformations when administered during pregnancy. Studies in animals have shown reproductive toxicity.
Letrozole Tablets USP, 2.5 mg is contraindicated during pregnancy.
Breast-feeding
It is unknown whether letrozole and its metabolites are excreted in human milk. A risk to the newborns/infants cannot be excluded.
Letrozole Tablets USP, 2.5 mg is contraindicated during breast-feeding.
Fertility
The pharmacological action of letrozole is to reduce oestrogen production by aromatase inhibition. In premenopausal women, the inhibition of oestrogen synthesis leads to feedback increases in gonadotropin (LH, FSH) levels. Increased FSH levels in turn stimulate follicular growth, and can induce ovulation.
Women of perimenopausal status or child-bearing potential
Letrozole Tablets USP, 2.5 mg should only be used in women with a clearly established postmenopausal status. As there are reports of women regaining ovarian function during treatment with Letrozole Tablets USP, 2.5 mg despite a clear postmenopausal status at start of therapy, the physician needs to discuss adequate contraception when necessary.
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
Letrozole Tablets USP, 2.5 mg has minor influence on the ability to drive and use machines. Since fatigue and dizziness have been observed with the use of Letrozole Tablets USP, 2.5 mg and somnolence has been reported uncommonly, caution is advised when driving or using machines.
SIDE EFFECTS
Infections and infestations
Uncommon: Urinary tract infection
Neoplasms, benign, malignant and unspecified (including cysts and polyps)
Uncommon: Tumour pain (not applicable in the adjuvant and extended adjuvant setting)
Blood and the lymphatic system disorders
Uncommon: Leukopenia
Metabolism and nutrition disorders
Common: Anorexia, appetite increase, hypercholesterolaemia
Uncommon: General oedema
Psychiatric disorders
Common: Depression
Uncommon: Anxiety including nervousness, irritability
Nervous system disorders
Common: Headache, dizziness
Uncommon: Somnolence, insomnia, memory impairment, dysaesthesia including paresthesia, hypoesthesia, taste disturbance, cerebrovascular accident
Eye disorders
Uncommon Cataract, eye irritation, blurred vision
Cardiac disorders
Uncommon: Palpitations, tachycardia
Vascular disorders
Uncommon: Thrombophlebitis including superficial and deep thrombophlebitis, hypertension, ischemic cardiac events
Rare: Pulmonary embolism, arterial thrombosis, cerebrovascular infarction
Respiratory, thoracic and mediastinal disorders
Uncommon: Dyspnoea, cough
Gastrointestinal disorders
Common: Nausea, vomiting, dyspepsia, constipation, diarrhoea
Uncommon: Abdominal pain, stomatitis, dry mouth
Hepatobiliary disorders
Uncommon: Increased hepatic enzymes
Skin and subcutaneous tissue disorders
Very common: Increased sweating
Common: Alopecia, rash including erythematous, maculopapular, psoriaform, and vesicular rash
Uncommon: Pruritus, dry skin, urticaria
Not known: Angioedema, anaphylactic reaction
Musculoskeletal and connective tissue disorders
Very common: Arthralgia
Common: Myalgia, bone pain, osteoporosis, bone fractures Uncommon: Arthritis
Renal and urinary disorders
Uncommon Increased urinary frequency
Reproductive system and breast disorders
Uncommon Vaginal bleeding, vaginal discharge, vaginal dryness, breast pain
General disorders and administration site conditions
Very common: Hot flushes, fatigue including asthenia
Common: Malaise, peripheral oedema
Uncommon: Pyrexia, mucosal dryness, thirst
Investigations
Common: Weight increase Uncommon: Weight loss
OVERDOSE
Isolated cases of overdose with Letrozole Tablets USP, 2.5 mg have been reported.
No specific treatment for overdose is known; treatment should be symptomatic and supportive.
PHARMACOLOGICALPROPERTIES
Pharmacotherapeutic group: Endocrine therapy. Hormone antagonist and related agents: aromatase inhibitor, ATC code: L02BG04.
Pharmacodynamic effects
The elimination of oestrogen-mediated growth stimulation is a prerequisite for tumour response in cases where the growth of tumour tissue depends on the presence of oestrogens and endocrine therapy is used. In postmenopausal women, oestrogens are mainly derived from the action of the aromatase enzyme, which converts adrenal androgens – primarily androstenedione and testosterone – to oestrone and oestradiol. The suppression of oestrogen biosynthesis in peripheral tissues and the cancer tissue itself can therefore be achieved by specifically inhibiting the aromatase enzyme.
Letrozole is a non-steroidal aromatase inhibitor. It inhibits the aromatase enzyme by competitively binding to the haem of the aromatase cytochrome P450, resulting in a reduction of oestrogen biosynthesis in all tissues where present.
In healthy postmenopausal women, single doses of 0.1 mg, 0.5 mg, and 2.5 mg letrozole suppress serum oestrone and oestradiol by 75%, 78% and 78% from baseline respectively. Maximum suppression is achieved in 48-78 hours.
In postmenopausal patients with advanced breast cancer, daily doses of 0.1 mg to 5 mg suppressed plasma concentration of oestradiol, oestrone, and oestrone sulphate by 75-95% from baseline in all patients treated. With doses of 0.5 mg and higher, many values of oestrone and oestrone sulphate were below the limit of detection in the assays, indicating that higher oestrogen suppression is achieved with these doses. Oestrogen suppression was maintained throughout treatment in all these patients.
Letrozole is highly specific in inhibiting aromatase activity. Impairment of adrenal steroidogenesis has not been observed. No clinically relevant changes were found in the plasma concentrations of cortisol, aldosterone, 11-deoxycortisol, 17- hydroxyprogesterone, and ACTH or in plasma renin activity among postmenopausal patients treated with a daily dose of letrozole 0.1 to 5 mg. The ACTH stimulation test performed after 6 and 12 weeks of treatment with daily doses of 0.1 mg, 0.25 mg, 0.5 mg, 1 mg, 2.5 mg, and 5 mg did not indicate any attenuation of aldosterone or cortisol production. Thus, glucocorticoid and mineralocorticoid supplementation is not necessary.
No changes were noted in plasma concentrations of androgens (androstenedione and testosterone) among healthy postmenopausal women after 0.1 mg, 0.5 mg, and 2.5 mg single doses of letrozole or in plasma concentrations of androstenedione among postmenopausal patients treated with daily doses of 0.1 mg to 5 mg, indicating that the blockade of oestrogen biosynthesis does not lead to accumulation of androgenic precursors. Plasma levels of LH and FSH are not affected by letrozole in patients, nor is thyroid function as evaluated by TSH, T4, and T3 uptake test.
Adjuvant treatment
Study BIG 1-98
BIG 1-98 was a multicentre, double-blind study in which over 8,000 postmenopausal women with hormone receptor- positive early breast cancer were randomised to one of the following treatments: A. tamoxifen for 5 years; B. Letrozole Tablets USP, 2.5 mg for 5 years; C. tamoxifen for 2 years followed by Letrozole Tablets USP, 2.5 mg for 3 years; D. Letrozole Tablets USP, 2.5 mg for 2 years followed by tamoxifen for 3 years.
The primary endpoint was disease-free survival (DFS); secondary efficacy endpoints were time to distant metastasis (TDM), distant disease-free survival (DDFS), overall survival (OS), systemic disease-free survival (SDFS), invasive contralateral breast cancer and time to breast cancer recurrence.
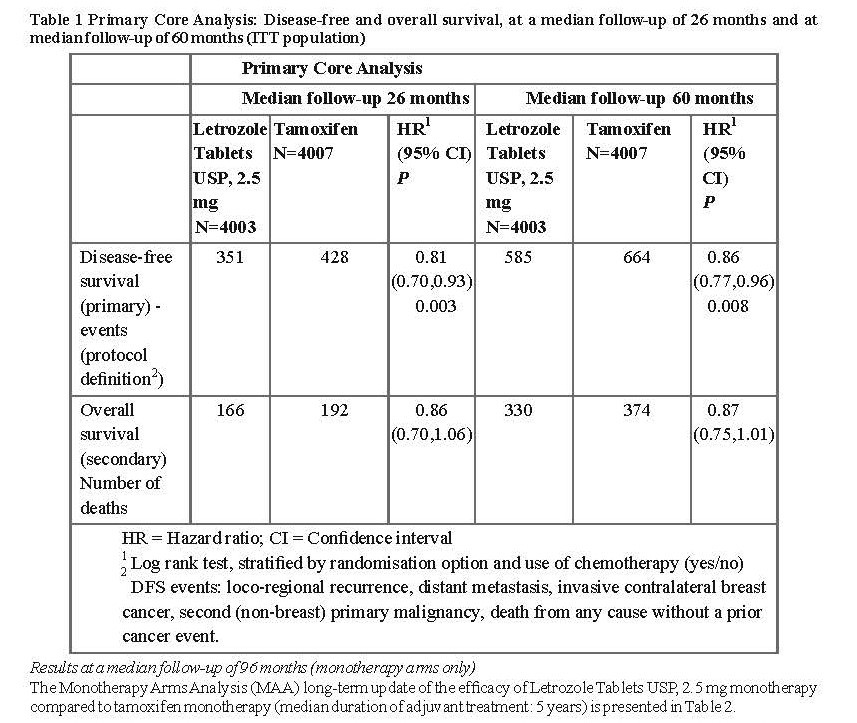
Efficacy results at a median follow-up of 26 and 60 months
Data in Table 1 reflect the results of the Primary Core Analysis (PCA) based on data from the monotherapy arms (A and B) and from the two switching arms (C and D) at a median treatment duration of 24 months and a median follow-up of 26 months and at a median treatment duration of 32 months and a median follow-up of 60 months.
The 5-year DFS rates were 84% for Letrozole Tablets USP, 2.5 mg and 81.4% for tamoxifen.
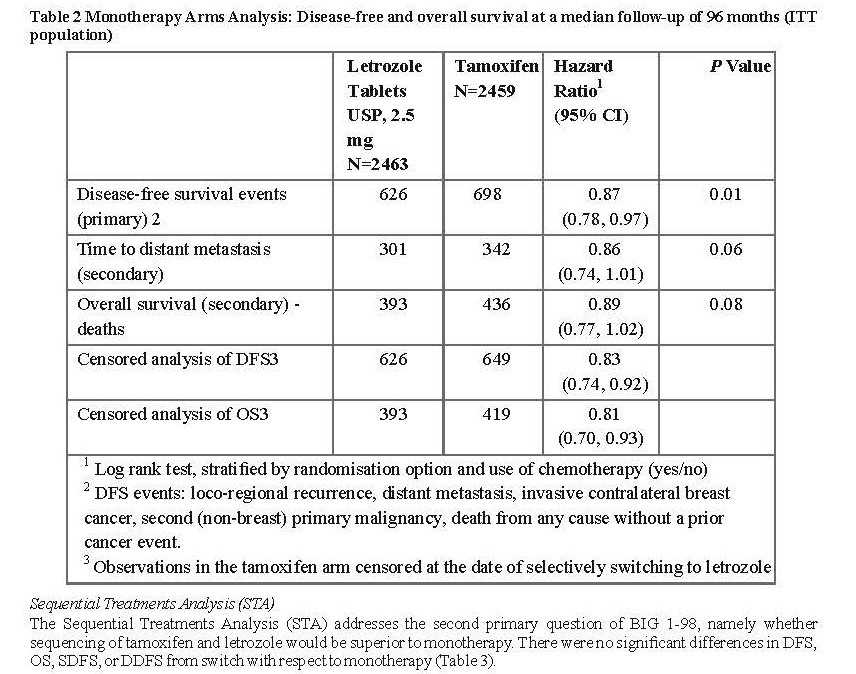
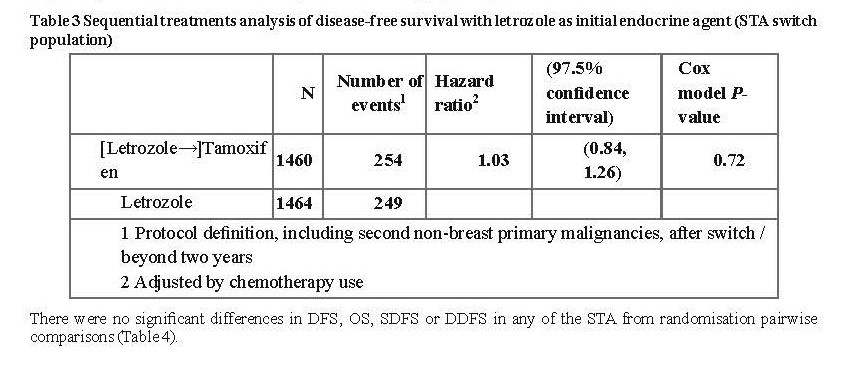
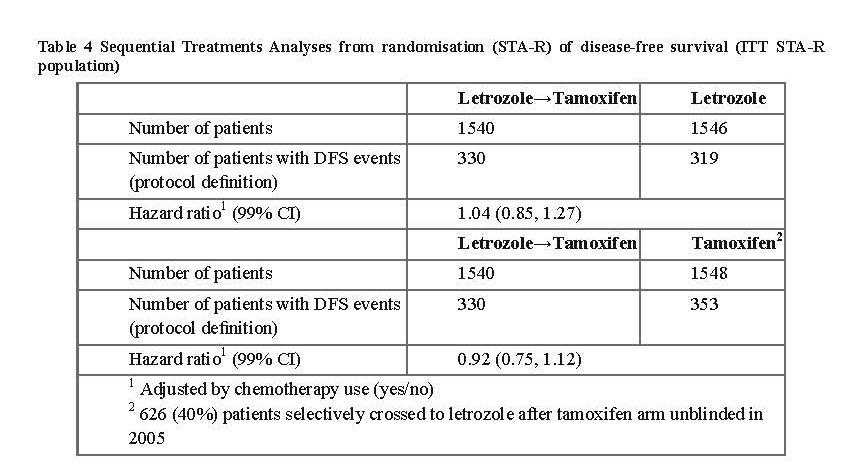
Study D2407
Study D2407 is an open-label, randomised, multicentre post approval safety study designed to compare the effects of adjuvant treatment with letrozole and tamoxifen on bone mineral density (BMD) and serum lipid profiles. A total of 262 patients were assigned either letrozole for 5 years or tamoxifen for 2 years followed by letrozole for 3 years.
At 24 months there was a statistically significant difference in the primary end-point; the lumbar spine BMD (L2-L4) showed a median decrease of 4.1% for letrozole compared to a median increase of 0.3% for tamoxifen.
No patient with a normal BMD at baseline became osteoporotic during 2 years of treatment and only 1 patient with osteopenia at baseline (T score of -1.9) developed osteoporosis during the treatment period (assessment by central review).
The results for total hip BMD were similar to those for lumbar spine but less pronounced.
There was no significant difference between treatments in the rate of fractures – 15% in the letrozole arm, 17% in the tamoxifen arm.
Median total cholesterol levels in the tamoxifen arm were decreased by 16% after 6 months compared to baseline and this decrease was maintained at subsequent visits up to 24 months. In the letrozole arm, total cholesterol levels were relatively stable over time, giving a statistically significant difference in favour of tamoxifen at each time point.
Extended adjuvant treatment (MA-17)
In a multicentre, double-blind, randomised, placebo-controlled study (MA-17), over 5,100 postmenopausal women with receptor-positive or unknown primary breast cancer who had completed adjuvant treatment with tamoxifen (4.5 to 6 years) were randomised to either Letrozole Tablets USP, 2.5 mg or placebo for 5 years.
The primary endpoint was disease-free survival, defined as the interval between randomisation and the earliest occurrence of loco-regional recurrence, distant metastasis, or contralateral breast cancer.
The first planned interim analysis at a median follow-up of around 28 months (25% of patients being followed up for at least 38 months), showed that Letrozole Tablets USP, 2.5 mg significantly reduced the risk of breast cancer recurrence by 42% compared with placebo (HR 0.58; 95% CI 0.45, 0.76; P=0.00003). The benefit in favour of letrozole was observed regardless of nodal status. There was no significant difference in overall survival: (Letrozole Tablets USP, 2.5 mg 51 deaths; placebo 62; HR 0.82; 95% CI 0.56, 1.19).
Consequently, after the first interim analysis the study was unblinded and continued in an open-label fashion and patients in the placebo arm were allowed to switch to Letrozole Tablets USP, 2.5 mg for up to 5 years. Over 60% of eligible patients (disease-free at unblinding) opted to switch to Letrozole Tablets USP, 2.5 mg. The final analysis included 1,551 women who switched from placebo to Letrozole Tablets USP, 2.5 mg at a median of 31 months (range 12 to 106 months) after completion of tamoxifen adjuvant therapy. Median duration for Letrozole Tablets USP, 2.5 mg after switch was 40 months.
The final analysis conducted at a median follow-up of 62 months confirmed the significant reduction in the risk of breast cancer recurrence with Letrozole Tablets USP, 2.5 mg.
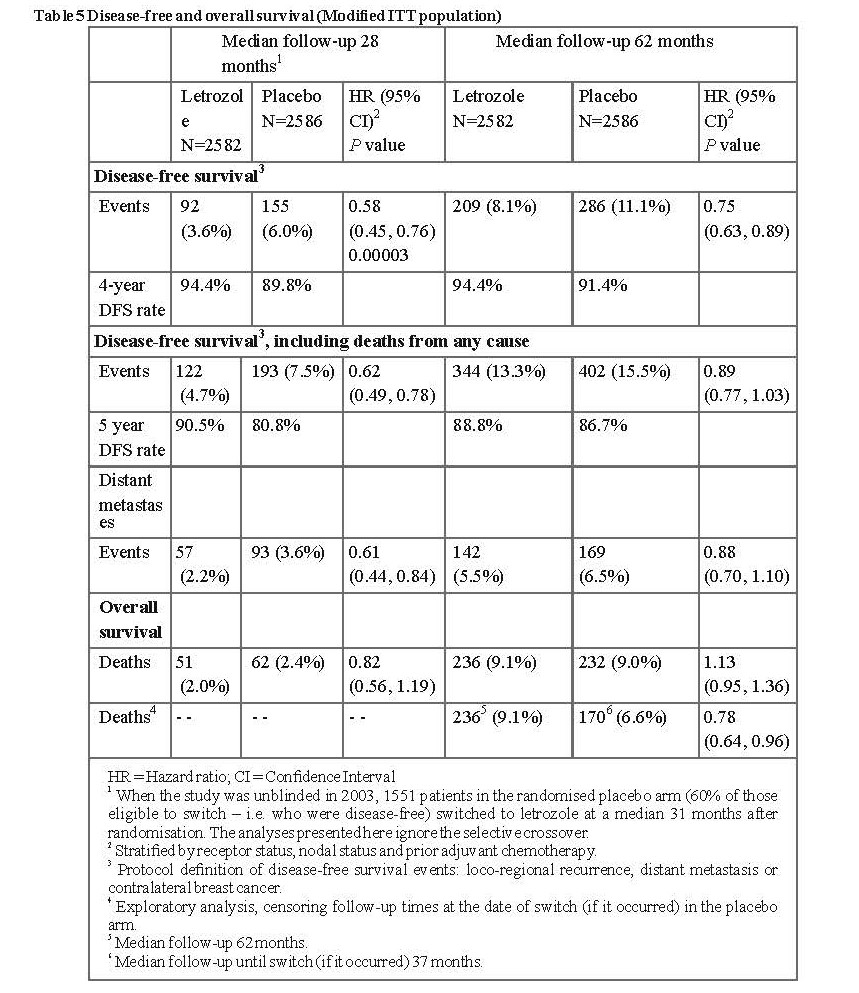
In the MA-17 bone substudy in which concomitant calcium and vitamin D were given, greater decreases in BMD compared to baseline occurred with Letrozole Tablets USP, 2.5 mg compared with placebo. The only statistically significant difference occurred at 2 years and was in total hip BMD (letrozole median decrease of 3.8% vs placebo median decrease of 2.0%).
In the MA-17 lipid substudy there were no significant differences between letrozole and placebo in total cholesterol or in any lipid fraction.
In the updated quality of life substudy there were no significant differences between treatments in physical component summary score or mental component summary score, or in any domain score in the SF-36 scale. In the MENQOL scale, significantly more women in the Letrozole Tablets USP, 2.5 mg arm than in the placebo arm were most bothered (generally in the first year of treatment) by those symptoms deriving from oestrogen deprivation – hot flushes and vaginal dryness. The symptom that bothered most patients in both treatment arms was aching muscles, with a statistically significant difference in favour of placebo.
Neoadjuvant treatment
A double blind trial (P024) was conducted in 337 postmenopausal breast cancer patients randomly allocated either Letrozole Tablets USP, 2.5 mg for 4 months or tamoxifen for 4 months. At baseline all patients had tumours stage T2-T4c, N0-2, M0, ER and/or PgR positive and none of the patients would have qualified for breast-conserving surgery. Based on clinical assessment there were 55% objective responses in the Letrozole Tablets USP, 2.5 mg arm versus 36% for the tamoxifen arm (P<0.001). This finding was consistently confirmed by ultrasound (Letrozole Tablets USP, 2.5 mg 35% vs tamoxifen 25%, P=0.04) and mammography (Letrozole Tablets USP, 2.5 mg 34% vs tamoxifen 16%, P<0.001). In total 45% of patients in the Letrozole Tablets USP, 2.5 mg group versus 35% of patients in the tamoxifen group (P=0.02) underwent breast-conserving therapy). During the 4-month pre-operative treatment period, 12% of patients treated with Letrozole Tablets USP, 2.5 mg and 17% of patients treated with tamoxifen had disease progression on clinical assessment.
First-line treatment
One controlled double-blind trial was conducted comparing Letrozole Tablets USP, 2.5 mg (letrozole) 2.5 mg to tamoxifen 20 mg as first-line therapy in postmenopausal women with advanced breast cancer. In 907 women, letrozole was superior to tamoxifen in time to progression (primary endpoint) and in overall objective response, time to treatment failure and clinical benefit.
The results are summarised in Table 6:
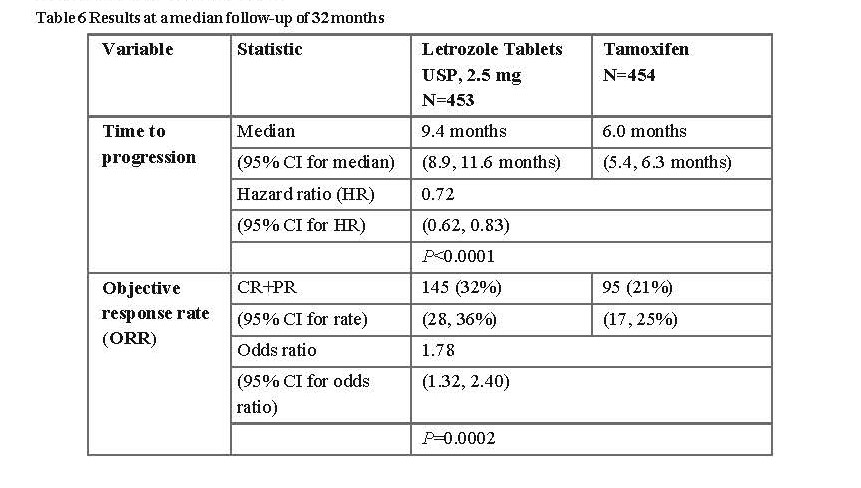
Time to progression was significantly longer, and response rate significantly higher for letrozole irrespective of whether adjuvant anti-oestrogen therapy had been given or not. Time to progression was significantly longer for letrozole irrespective of dominant site of disease. Median time to progression was 12.1 months for Letrozole Tablets USP, 2.5 mg and 6.4 months for tamoxifen in patients with soft tissue disease only and median 8.3 months for Letrozole Tablets USP,
2.5 mg and 4.6 months for tamoxifen in patients with visceral metastases.
Study design allowed patients to cross over upon progression to the other therapy or discontinue from the study. Approximately 50% of patients crossed over to the opposite treatment arm and crossover was virtually completed by 36 months. The median time to crossover was 17 months (Letrozole Tablets USP, 2.5 mg to tamoxifen) and 13 months (tamoxifen to Letrozole Tablets USP, 2.5 mg).
Letrozole Tablets USP, 2.5 mg treatment in the first-line therapy of advanced breast cancer resulted in a median overall survival of 34 months compared with 30 months for tamoxifen (logrank test P=0.53, not significant). The absence of an advantage for Letrozole Tablets USP, 2.5 mg on overall survival could be explained by the crossover design of the study.
Second-line treatment
Two well-controlled clinical trials were conducted comparing two letrozole doses (0.5 mg and 2.5 mg) to megestrol acetate and to aminoglutethimide, respectively, in postmenopausal women with advanced breast cancer previously treated with anti-oestrogens.
Time to progression was not significantly different between letrozole 2.5 mg and megestrol acetate (P=0.07). Statistically significant differences were observed in favour of letrozole 2.5 mg compared to megestrol acetate in overall objective tumour response rate (24% vs 16%, P=0.04), and in time to treatment failure (P=0.04). Overall survival was not significantly different between the 2 arms (P=0.2).
In the second study, the response rate was not significantly different between letrozole 2.5 mg and aminoglutethimide (P=0.06). Letrozole 2.5 mg was statistically superior to aminoglutethimide for time to progression (P=0.008), time to treatment failure (P=0.003) and overall survival (P=0.002).
Male breast cancer
Use of Letrozole Tablets USP, 2.5 mg in men with breast cancer has not been studied.
Pharmacokinetic properties
Absorption
Letrozole is rapidly and completely absorbed from the gastrointestinal tract (mean absolute bioavailability: 99.9%). Food slightly decreases the rate of absorption (median tmax 1 hour fasted versus 2 hours fed; and mean Cmax 129 ± 20.3 nmol/litre fasted versus 98.7 ± 18.6 nmol/litre fed) but the extent of absorption (AUC) is not changed. The minor effect on the absorption rate is not considered to be of clinical relevance, and therefore letrozole may be taken without regard to mealtimes.
Distribution
Plasma protein binding of letrozole is approximately 60%, mainly to albumin (55%). The concentration of letrozole in erythrocytes is about 80% of that in plasma. After administration of 2.5 mg 14C-labelled letrozole, approximately 82% of the radioactivity in plasma was unchanged compound. Systemic exposure to metabolites is therefore low. Letrozole is rapidly and extensively distributed to tissues. Its apparent volume of distribution at steady state is about 1.87 ± 0.47 l/kg.
Biotransformation
Metabolic clearance to a pharmacologically inactive carbinol metabolite is the major elimination pathway of letrozole (CLm = 2.1 l/h) but is relatively slow when compared to hepatic blood flow (about 90 l/h). The cytochrome P450 isoenzymes 3A4 and 2A6 were found to be capable of converting letrozole to this metabolite. Formation of minor unidentified metabolites and direct renal and faecal excretion play only a minor role in the overall elimination of letrozole. Within 2 weeks after administration of 2.5 mg 14C-labelled letrozole to healthy postmenopausal volunteers, 88.2 ± 7.6% of the radioactivity was recovered in urine and 3.8 ± 0.9% in faeces. At least 75% of the radioactivity recovered in urine up to 216 hours (84.7 ± 7.8% of the dose) was attributed to the glucuronide of the carbinol metabolite, about 9% to two unidentified metabolites, and 6% to unchanged letrozole.
Elimination
The apparent terminal elimination half-life in plasma is about 2 to 4 days. After daily administration of 2.5 mg steady-state levels are reached within 2 to 6 weeks. Plasma concentrations at steady state are approximately 7 times higher than concentrations measured after a single dose of 2.5 mg, while they are 1.5 to 2 times higher than the steady-state values predicted from the concentrations measured after a single dose, indicating a slight non-linearity in the pharmacokinetics of letrozole upon daily administration of 2.5 mg. Since steady-state levels are maintained over time, it can be concluded that no continuous accumulation of letrozole occurs.
Linearity/non-linearity
The pharmacokinetics of letrozole were dose proportional after single oral doses up to 10 mg (dose range: 0.01 to 30 mg) and after daily doses up to 1.0 mg (dose range: 0.1 to 5mg). After a 30 mg single oral dose there was a slightly dose over- proportional increase in AUC value. The dose over-proportionality is likely to be the result of a saturation of metabolic elimination processes. Steady levels were reached after 1 to 2 months at all dosage regimens tested (0.1-5.0 mg daily).
Special populations
Elderly
Age had no effect on the pharmacokinetics of letrozole.
Renal impairment
In a study involving 19 volunteers with varying degrees of renal function (24-hour creatinine clearance 9-116 ml/min) no effect on the pharmacokinetics of letrozole was found after a single dose of 2.5 mg. In addition to the above study assessing the influence of renal impairment on letrozole, a covariate analysis was performed on the data of two pivotal studies (Study AR/BC2 and Study AR/BC3). Calculated creatinine clearance (CLcr) [Study AR/BC2 range: 19 to 187 mL/min; Study AR/BC3 range: 10 to 180 mL/min] showed no statistically significant association between letrozole plasma trough levels at steady-state (Cmin). Futhermore, data of Study AR/BC2 and Study AR/BC3 in second-line metastatic breast cancer showed no evidence of an adverse effect of letrozole on CLcr or an impairment of renal function. Therefore, no dose adjustment is required for patients with renal impairment (CLcr ≥10 mL/min). Little information is available in patients with severe impairment of renal function (CLcr <10 mL/min).
Hepatic impairment
In a similar study involving subjects with varying degrees of hepatic function, the mean AUC values of the volunteers with moderate hepatic impairment (Child-Pugh B) was 37% higher than in normal subjects, but still within the range seen in subjects without impaired function. In a study comparing the pharmacokinetics of letrozole after a single oral dose in eight male subjects with liver cirrhosis and severe hepatic impairment (Child-Pugh C) to those in healthy volunteers (N=8), AUC and t½ increased by 95 and 187%, respectively. Thus, Letrozole Tablets USP, 2.5 mg should be administered with caution to patients with severe hepatic impairment and after consideration of the risk/benet in the individual patient.
PHARMACEUTICAL PARTICULARS
List of excipients
Lactose Monohydrate
Microcrystalline Cellulose (Comprecel M 101 D+)
Corn Starch
Sodium Starch Glycolate
Magnesium Stearate
Colloidal Silica dioxide
Hypromellose
Polyethylene glycol
Talc
Macrogol 8000
Titanium Dioxide
Yellow Iron Oxide
Shelf life: 2 years
Storage: Store in a cool & dry place below 30°C.
Protect from light & moisture.
Nature and contents of container:
PVC/PE/PVdC triple laminated clear film and Aluminium foil, blister packs of 3×10 tablets contained in a carton.
HDPE bottle packs of 30 tablets in a carton.
Manufactured by:
M/s Jodas Expoim Pvt. Ltd.
Plot No.55, Phase III, Biotech Park,
Karkapatla (V), Markook (M),
Siddipet (D)-502 279,
Telangana INDIA.
Product of:
Grace Biogen PtyLtd,
Sydney, Australia.